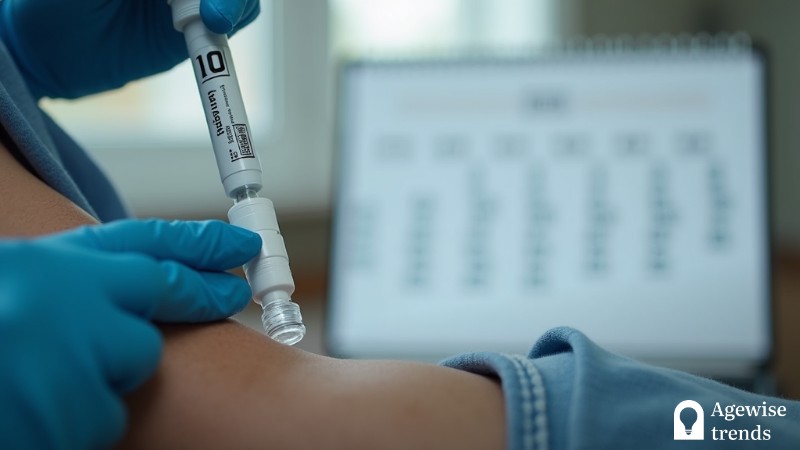
Following its review process, the US Food and Drug Administration has approved the use of Leqembi, a medication co-developed by Biogen and Eisai. This treatment involves administering monthly doses to maintain its effectiveness in patients with Alzheimer’s.
The approval is expected to provide a significant sales boost for the anti-amyloid therapy, which has faced slow growth due to its time-consuming administration and the need for regular screenings.
After receiving Leqembi through an IV twice a month for 18 months, patients will have the option to switch to once-a-month doses or continue with their current treatment schedule.
Key Takeaways
The US FDA has approved Leqembi, a medication co-developed by Biogen and Eisai, for monthly maintenance dosing in patients with Alzheimer’s disease.
- Leqembi slows the progression of cognitive decline in individuals with early-stage Alzheimer’s, making it a significant option in managing the disease.
- The FDA approval allows patients to switch from bi-weekly IV infusions to once-a-month doses or continue their current treatment schedule after 18 months.
- Despite FDA approval, slow sales growth presents challenges in Leqembi’s adoption among healthcare providers due to reimbursement issues and lack of awareness about continued treatment.
FDA approval and data behind Leqembi’s efficacy
The FDA’s approval of Leqembi was based on modeling findings that used data from clinical trials and simulations, which predicted that maintenance dosing would effectively sustain the treatment’s benefits.
The continued effectiveness of this treatment is evident, especially when considering that the progression of Alzheimer’s disease persists even after amyloid plaques are removed.
Leqembi has been shown to slow the progression of cognitive decline in individuals with early-stage Alzheimer’s, making it a significant option in managing the disease.
The clearance also highlights the convenience of maintenance dosing, which may be easier for both patients and their caregivers to manage over time.
In addition to its intravenous form, Eisai and Biogen are developing a more convenient injectable version of the drug to further enhance treatment options.
Typically, patients discontinue the medication once brain scans show no signs of amyloid plaque buildup. However, certain treatments, including Leqembi, carry warnings regarding the potential for increased intracranial pressure and hemorrhaging, with the FDA recommending regular scans to monitor these risks.
Eisai and BioArctic have formed a partnership under an agreement to co-develop the drug, aiming to bring the treatment to a broader patient base.
Memory loss and dementia care
The green light is particularly significant, as Alzheimer’s disease affects nearly seven million people in the US, leading to irreversible and progressive neurodegeneration.
Leqembi works by binding to amyloid beta protein aggregates, preventing their transformation into harmful plaques in the brain.
Despite this breakthrough, challenges persist, as slow sales growth highlights concerns around reimbursement barriers and the need for greater awareness among healthcare providers.
Analysts have identified several factors contributing to the struggles with the treatment’s uptake, including reimbursement difficulties and a lack of sufficient awareness regarding proper protocols.
Understanding Leqembi
Leqembi is a drug designed to target amyloid plaques in the brains of individuals with Alzheimer’s disease. In July 2023, it received standard approval in the United States after demonstrating its ability to slow mental decline in those with mild Alzheimer’s.
After 18 months of bi-weekly IV infusions, patients can transition to maintenance therapy with less frequent doses or continue with regular fortnightly injections. This flexibility may help make ongoing treatment more manageable for both patients and caregivers.
For those using the injectable form, the reduced frequency of treatments could also lessen the need for frequent hospital visits and extensive nursing support, making the overall experience more convenient.
Overcoming challenges to effective treatment
Despite FDA approval, slow sales growth presents challenges in Leqembi’s adoption among healthcare providers.
Key obstacles include reimbursement issues and a lack of awareness about the importance of continued treatment with the medication—factors analysts believe must be addressed to encourage broader acceptance.
Reimbursement challenges, in particular, hinder access for eligible patients, requiring proactive solutions to ensure ongoing treatment availability.
To overcome these barriers, Biogen and Eisai must focus on both addressing reimbursement concerns and raising awareness among healthcare providers.
A strong commitment to educating healthcare professionals and patients about the importance of prolonged treatment will be crucial for fostering greater uptake and ensuring the medication’s long-term success.