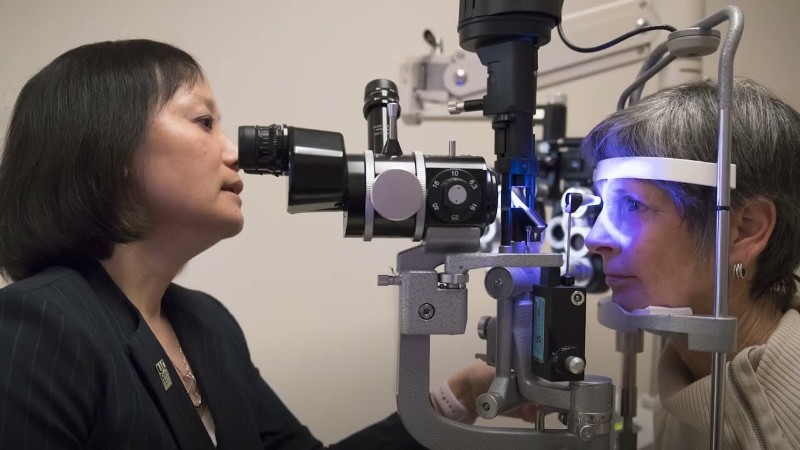
The U.S. Food and Drug Administration (FDA) has granted approval for Susvimo (ranibizumab injection) 100 mg/mL to treat diabetic macular edema (DME), a condition that can lead to significant vision loss in individuals with diabetes. Developed by Genentech, a Roche Group company, Susvimo is the first continuous drug delivery system designed to reduce the need for frequent eye injections, making treatment more convenient for patients.
This approval marks Susvimo’s second FDA indication, as it was previously approved in 2021 for treating wet (neovascular) age-related macular degeneration (nAMD). By offering an alternative to monthly anti-VEGF injections, Susvimo provides a significant breakthrough in long-term vision preservation for DME patients.
Key Takeaways
FDA approves Susvimo, a new continuous drug delivery system designed to treat diabetic macular edema (DME), offering patients fewer injections compared to traditional treatments.
- Susvimo is the first refillable implant that delivers ranibizumab for up to six months, reducing the need for frequent eye injections.
- Clinical trials show that Susvimo provides vision improvements comparable to monthly anti-VEGF injections with significantly fewer treatments.
- Genentech offers support programs including insurance coverage assistance and affordability programs to ensure broader patient access.
New approach to treating diabetic macular edema
DME is a primary cause of vision loss in adults with diabetes, impacting almost 29 million individuals globally. It occurs when damaged blood vessels leak fluid into the retina, causing swelling in the macula, which is responsible for sharp central vision. Without treatment, DME can lead to progressive vision impairment or blindness.
The standard treatment for DME involves frequent eye injections of anti-VEGF drugs, which help reduce swelling and slow disease progression. However, many patients find the burden of monthly injections challenging due to scheduling conflicts, travel difficulties, and needle-related anxiety. The introduction of Susvimo as a refillable implant offers a more patient-friendly solution with fewer required treatments.
Clinical trial success and FDA approval
The FDA’s approval of Susvimo is based on one-year results from the Phase III Pagoda study, which evaluated its safety and effectiveness in people with DME. The study found that patients receiving Susvimo refills every six months experienced vision improvements comparable to those receiving monthly ranibizumab injections.
Patients using Susvimo gained an average of 9.6 letters on an eye chart, while those receiving monthly injections gained 9.4 letters. These results confirm that Susvimo provides sustained benefits with significantly fewer treatments.
“Susvimo presents a unique, convenient alternative to routine eye injections for people with a potentially blinding diabetic eye condition,” said Dr. Levi Garraway, Roche’s Chief Medical Officer and Head of Global Product Development.
How Susvimo works and its benefits
Continuous drug delivery through an eye implant: Susvimo is a small, refillable eye implant that delivers a customized formulation of ranibizumab, a drug that blocks vascular endothelial growth factor (VEGF-A)—a key protein responsible for abnormal blood vessel growth and leakage in the retina. Unlike traditional treatments that require monthly injections, Susvimo provides continuous drug delivery for up to six months, reducing the need for frequent doctor visits.
The implant is surgically placed into the eye in a one-time outpatient procedure and is refilled every six months. By maintaining consistent drug levels, it helps stabilize vision and prevent disease progression.
Improving patient experience: For many patients, reducing the number of treatments can lead to greater adherence to therapy, improved quality of life, and better long-term vision outcomes.
Dr. Jordan Graff, a vitreoretinal surgeon at Barnet Dulaney Perkins Eye Center in Arizona, emphasized the benefits of Susvimo, stating that he has seen firsthand how continuous delivery of medication can help preserve vision with fewer treatments. He also noted that this option is especially beneficial for patients with busy personal and professional lives who find it difficult to keep up with frequent injections.
Genentech’s commitment to expanding access
To ensure broader patient access to Susvimo, Genentech has introduced comprehensive support programs. The company is offering insurance coverage assistance to help patients and healthcare providers navigate reimbursement challenges. Additionally, affordability programs will be available for eligible patients to reduce financial barriers to treatment.
To support the safe and effective use of the implant, Genentech is also providing specialized training for retina specialists, ensuring that more doctors are equipped to offer this treatment to their patients. With this FDA approval, Susvimo is now available in the U.S., providing a more convenient and effective treatment option for people with diabetic macular edema.